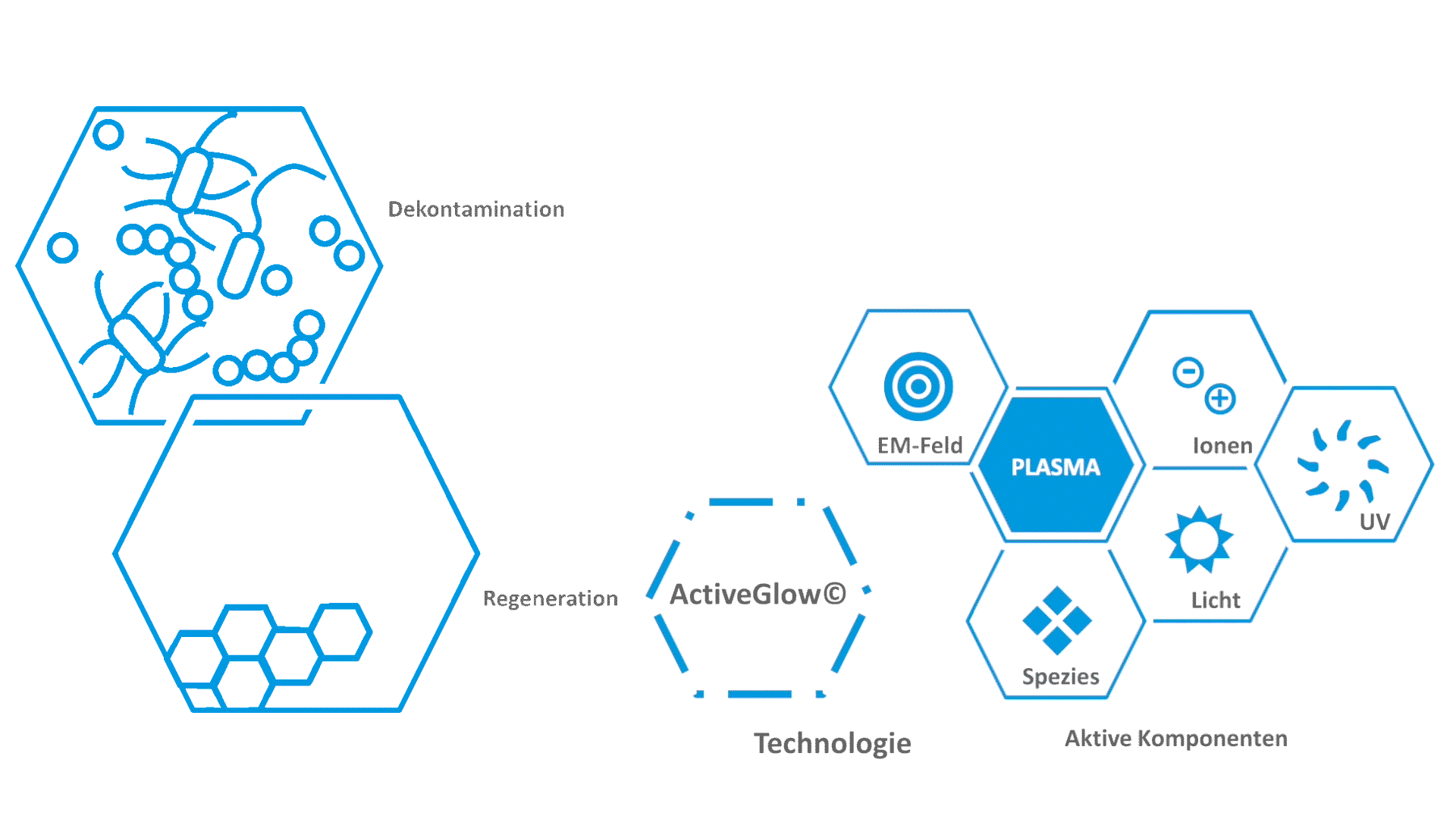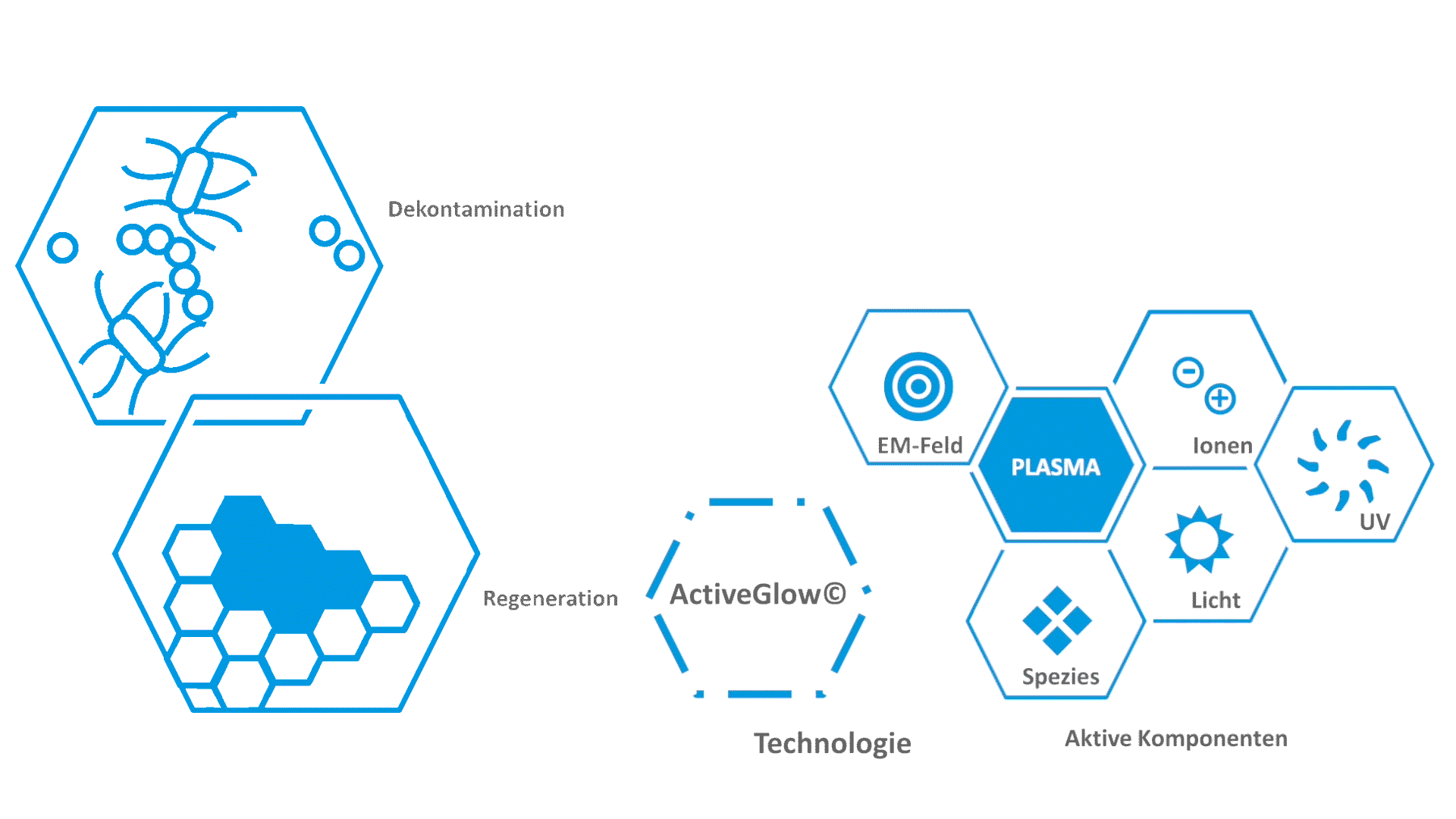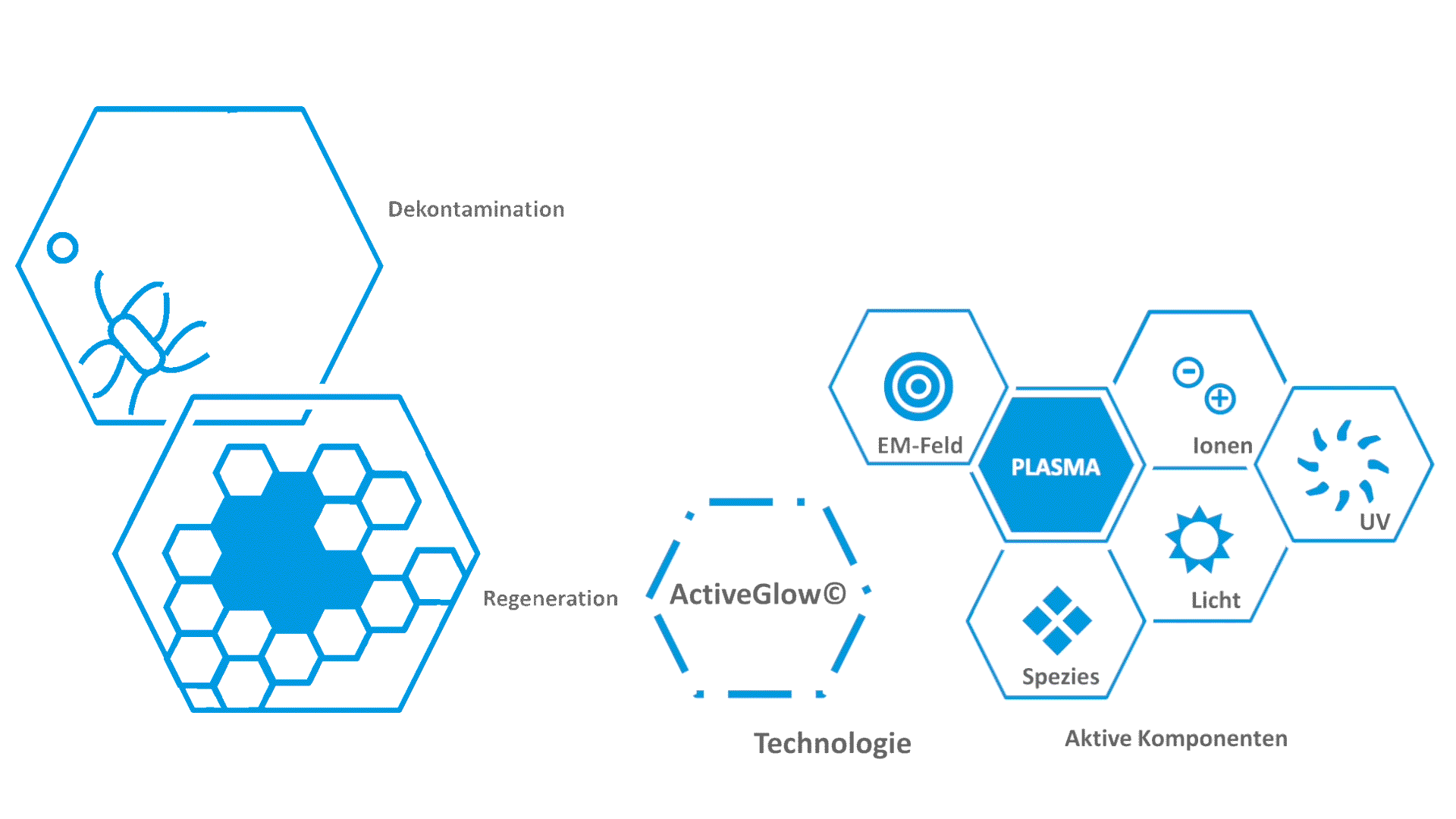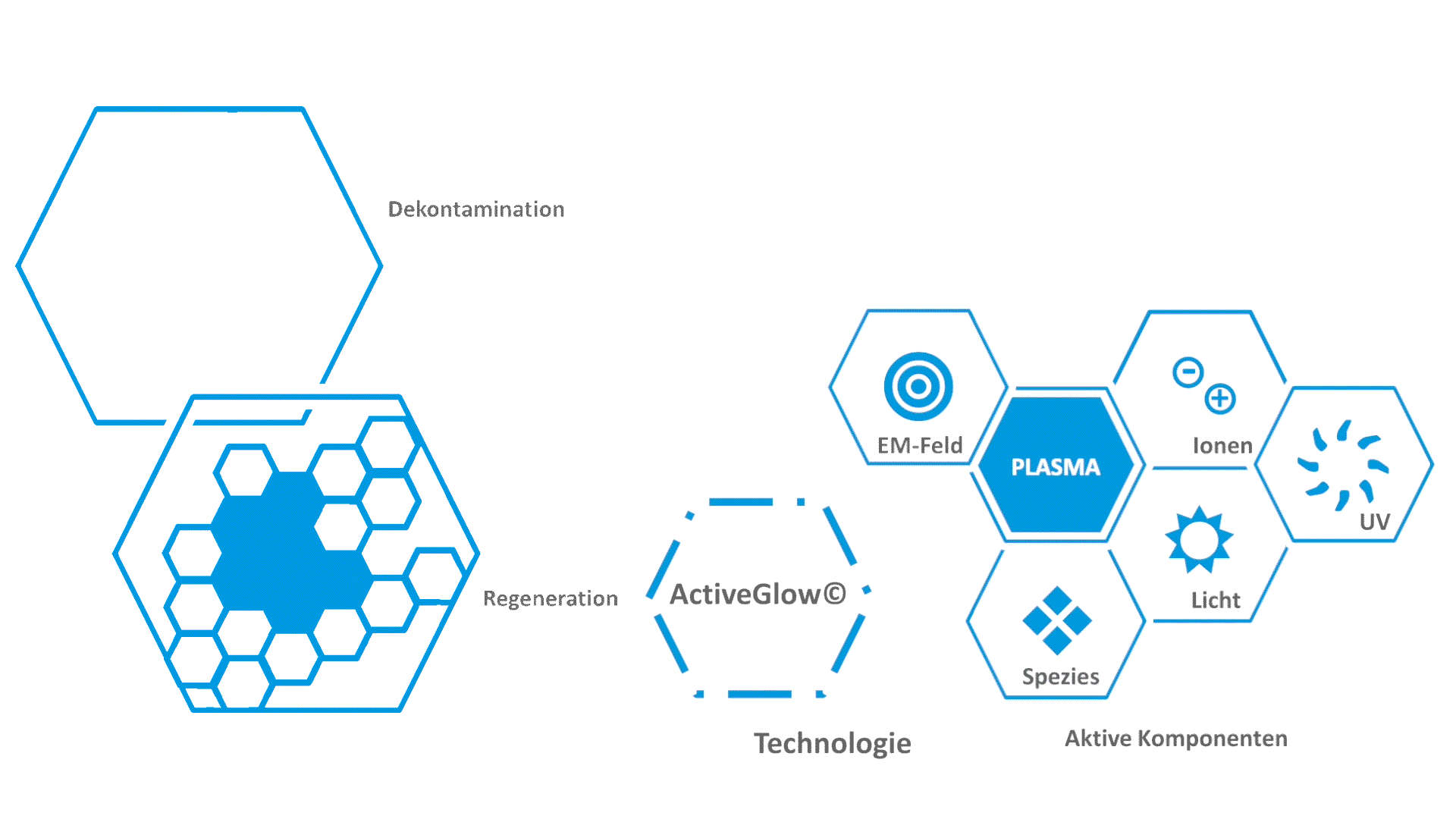
[1] M. Klebes, C. Ulrich, F. Kluschke, A. Patzelt, S. Vandersee, H. Richter, A. Bob, J. Hutten, J.T. Krediet, A. Kramer, Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment, Journal of biophotonics, 8 (2015) 382-391.
[2] F. Brehmer, H. Haenssle, G. Daeschlein, R. Ahmed, S. Pfeiffer, A. Görlitz, D. Simon, M. Schön, D. Wandke, S. Emmert, Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622), Journal of the European Academy of Dermatology and Venereology, 29 (2015) 148-155.
[3] S. Vandersee, H. Richter, J. Lademann, M. Beyer, A. Kramer, F. Knorr, B. Lange-Asschenfeldt, Laser scanning microscopy as a means to assess the augmentation of tissue repair by exposure of wounds to tissue tolerable plasma, Laser Physics Letters, 11 (2014) 115701.
[4] C. Ulrich, F. Kluschke, A. Patzelt, S. Vandersee, V. Czaika, H. Richter, A. Bob, J. von Hutten, C. Painsi, R. Hügel, Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study, Journal of wound care, 24 (2015).
[5] G. Isbary, W. Stolz, T. Shimizu, R. Monetti, W. Bunk, H.U. Schmidt, G.E. Morfill, T.G. Klämpfl, B. Steffes, H.M. Thomas, J. Heinlin, S. Karrer, M. Landthaler, J.L. Zimmermann, Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: results of an open retrospective randomized controlled study in vivo, Clinical Plasma Medicine, 1 (2013) 25-30.
[6] J. Heinlin, J.L. Zimmermann, F. Zeman, W. Bunk, G. Isbary, M. Landthaler, T. Maisch, R. Monetti, G. Morfill, T. Shimizu, J. Steinbauer, W. Stolz, S. Karrer, Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites, Wound Repair Regen, 21 (2013) 800-807.
[7] T. Von Woedtke, H.R. Metelmann, K.D. Weltmann, Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma, Contributions to Plasma Physics, 54 (2014) 104-117.
[8] J. Lademann, H. Richter, A. Alborova, D. Humme, A. Patzelt, A. Kramer, K.-D. Weltmann, B. Hartmann, C. Ottomann, J.W. Fluhr, Risk assessment of the application of a plasma jet in dermatology, Journal of biomedical optics, 14 (2009) 054025-054025-054026.